THE USE OF GLAUCONITE SANDS OF THE MABIKAN DEPOSIT TO IMPROVE THE PROPERTIES OF AMMONIUM NITRATE
KODIROV BEKZOD KHOMIDZHONOVICH, doctoral student
TOZHIEV RUSTAM RASULOVICH, Dean
FERGANA POLYTECHNIC INSTITUTE, UZBEKISTAN
e-mail: b.qodirov@ferpi.uz, Rustamjontojiyev658@gmail.com
Annotation. Ammonium nitrate is used both as a fertilizer in agriculture and as an ingredient for industrial explosives (for quarries, mine tunnels, etc.). The substance is stable under normal conditions of use, storage and transportation, but despite this stability, in the last century there were several accidents associated with a large number of ammonium nitrate, which has led to numerous deaths and injuries to people. This article analyzes in more detail the incidents related to the explosion of ammonium nitrate in the middle of the XX century (partially). Based on the official report on the conditions and consequences of the accident, various scenarios were considered using different TNT equivalence coefficients and the physical consequences of the explosion were determined.
Keywords: ammonium nitrate, properties, explosivity, hygroscopicity, porosity and particle density, qualitative indicators.
Introduction. Ammonium nitrate is an inorganic product of industrial synthesis, produced from gaseous ammonia and nitric acid. It was first obtained by Glauber in 1659. In its production form, it is a colorless crystalline substance with a content of 35% of total nitrogen in ammonia and nitrate forms. For fertilizing purposes, it is produced in granular form [1]. The physico-chemical properties of ammonium nitrate are regulated by the regulatory requirements of GOST 2-2013 [2,3].
Ammonium nitrate is well soluble in water. Its water solubility at 25oC exceeds 2 kg/kg of water, which is significantly higher than the solubility of many organic and inorganic compounds [4].
One of the important distinguishing characteristics of ammonium nitrate is its strong oxidizing ability in relation to a large number of inorganic and organic substances, which strongly affects its consumer and commodity properties. In recent years, explosions and fires caused by this characteristic of ammonium nitrate in a number of countries [5-7] have significantly reduced the demand for this important universal fertilizer products.
A characteristic specific indicator of ammonium nitrate is the polymorphism of its properties. This means its ability to change its crystal structure depending on the temperature of the medium. As is known [4,8-10], depending on temperature conditions, ammonium nitrate has five crystalline forms. At the same time, the structural transition in its composition from one crystal structure to another takes place under conditions of heat release or absorption, and leads to changes in the volume and density of crystals, as well as all its other physico-chemical properties. It has been established [11-14] that the III structural modification of ammonium nitrate is the most stable in relation to its transportation and storage in hot climatic conditions, a number of sufficiently effective ways of stabilizing its III structural modification have been found and developed [15-20].
Ammonium nitrate has a high traceability [3.21]. Because of this, in the practical application of ammonium nitrate as a fertilizer, there are significant losses of marketable products, additional costs are required when using the accumulated part of it [1,4]. As is known [4,22,23], the traceability of ammonium nitrate is caused by its high solubility, hygroscopicity, low strength of granules, as well as polymorphic transformations taking place in its structure. Therefore, the lower the solubility and hygroscopicity, the higher the strength of ammonium nitrate granules, the lower its traceability.
An important characteristic of the production ammonium nitrate is the mechanical strength of its granules. According to modern requirements [3], ammonium nitrate granules during transportation, storage and use should not be destroyed under mechanical loads. The strength of granules is characterized by three indicators: dynamic strength (Rd), abrasion resistance (At), static strength (Rd) [24,25]. The strength of ammonium nitrate granules is most significantly influenced by humidity, porosity and the size of the product granules [22,26,27]. With a decrease in humidity and the size of individual granules, as well as with an increase in their density, the strength of ammonium nitrate granules increases.
Ammonium nitrate, as a result of intensive moisture absorption, reduces its mechanical strength, collapses and cures [1,4, 28-30]. All this is due to its hygroscopicity. Because of this, the production ammonium nitrate needs to comply with special storage conditions, causes a number of difficulties in its practical use for fertilizing purposes [31-36].
Therefore, today everywhere both manufacturers of ammonium nitrate and its consumers are keenly interested in reducing its hygroscopicity by various methods and techniques [37-42].
The world practice of using ammonium nitrate indicates that it is characterized by flammability and explosiveness [45-48]. This is due to the fact that ammonium nitrate is prone to thermal decomposition and has strong oxidizing properties. Thermal decomposition of ammonium nitrate takes place with slow heating starting from 110oC and above. At low temperatures, the products of thermal decomposition are ammonia and nitric acid, and at high temperatures, nitrous oxide, nitrogen oxides and oxygen [4,49-51]. At the same time, in the high temperature region, the formation of the last products is explosive. Consequently, due to the thermal decomposition of ammonium nitrate, there is a loss of part of the product, as well as the practical use of ammonium nitrate becomes unsafe. Therefore, in order to completely suppress the process of thermal decomposition of ammonium nitrate or its effective deceleration, a number of methods and techniques for its stabilization are used in practice [52-55].
Ammonium nitrate is segmented by application (fertilizers, explosives and other applications), end users (agriculture, mining, defense and others) and geography (Asia-Pacific region, North America, Europe, South America, Middle East and Africa)). The report suggests the market size and forecasts of the ammonium nitrate market in volumes (kilotons) for all of the above segments.
The agricultural segment accounts for the main market share of ammonium nitrate.
In agriculture, fertilizers are used to enrich plant growth. They contain essential nutrients for plants that ensure healthy growth and protect them from diseases. There are various types of fertilizers that are available in soluble, dry crystalline forms and forms of prolonged action. Ammonium nitrate is one of such fertilizers, which is used to provide plants with a sufficient amount of nitrogen.
The ammonia fraction of ammonium nitrate is absorbed by the roots or gradually converted into nitrate by soil microorganisms. Many vegetable growers prefer an affordable nitrate plant food source and use ammonium nitrate. Livestock farmers like it for fertilizing pastures and hay, because it is less susceptible to evaporation losses than urea-based fertilizers when it remains on the soil surface.
Ammonium nitrate is usually mixed with other fertilizers, but these mixtures cannot be stored for a long time due to the tendency to absorb moisture from the air.
However, the explosion of 2,700 tons of ammonium nitrate in Beirut in 2020 demonstrates the importance of handling this substance and the need for stricter and clearly defined rules in the industry.
Consequently, due to the rapidly growing agricultural activity worldwide, the demand for solid ammonium nitrate is expected to grow rapidly during the forecast period (58).
Approximately half (50.5%) of the total global consumption of ammonium nitrate is accounted for by such major and developed countries of the world as the USA (17%), the Russian Federation (14%), France (5%), Brazil (2.5%), the UK (2%), and the rest is the share of all other countries of the world (Figure 1). Visual changes in the export and import of ammonium nitrate in physical terms are shown in Figure 2.
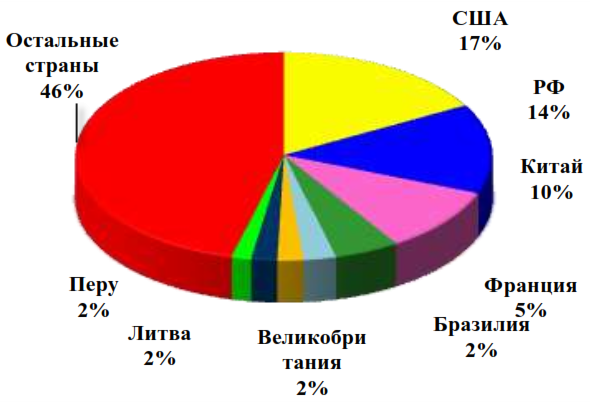
Source: IFA, Fertecon, company assessment [57]
Figure 1 – Structure of global consumption of ammonium nitrate in 2019 by leading countries of the world
In the CIS countries, the leaders in the market of nitrogen fertilizers, including ammonium nitrate, are the Russian Federation, Ukraine, Uzbekistan, Belarus and Kazakhstan, where today there are more than 28 modern production facilities [56,57].
There are currently 20 leading manufacturers and companies of ammonium nitrate in the world. Figure 2 shows that the Norwegian company “Yara” confidently leads in terms of production of ammonium nitrate, followed by three Russian companies “Uralchem”, “EuroChem”, “Akron” and Uzbek “Uzkimesanoat”.
Since 2008, restrictions on the supply of ammonium nitrate containing more than 28% nitrogen have been in effect in European countries. As a result, the export of domestic saltpeter to Europe, containing 34% nitrogen, was closed. In our country, certain measures are also being taken to restrict the export of ammonium nitrate to countries where military operations are underway or the domestic political situation is characterized by a high level of terrorist activity.
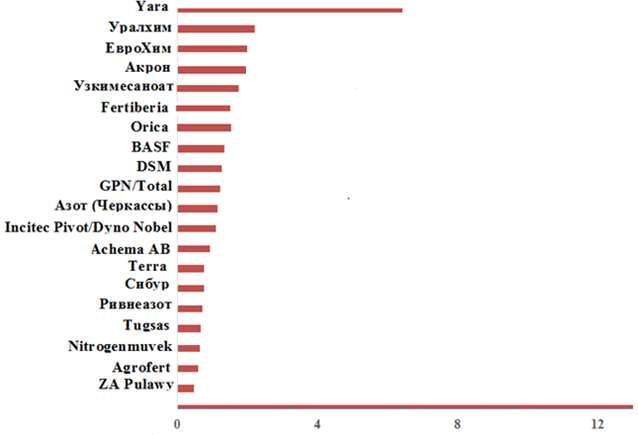
Source: based on the materials of the website www.uralchem.ru
Figure 2 – Dynamics of the volume of output of the sales market of 20 leading world manufacturers of ammonium nitrate, million tons
In this regard, the search for rational ways to reduce the explosive hazard of ammonium nitrate and increase its agrochemical characteristics is of great practical interest. The successful solution of these problems can help to increase the competitiveness of mineral fertilizers produced in our country and more actively promote them to the world market.
Currently, additives of limestone, chalk, chloride or potassium sulfate and some other mineral components are most often used to reduce the explosive hazard of ammonium nitrate (Kropacheva, RF).
The aim of the work was to study the possibility and feasibility of granulating ammonium nitrate in a mixture with glauconite sand from the Mabikan glauconite mine (Dehkanabad district of Kashkadarya region).
The choice of an additive is due to the following factors:
– glauconites are good sorbents capable of absorbing and retaining gases released during the decomposition of ammonium nitrate (reducing the threat of explosion);
– glauconite sands have a complex of valuable agrochemical properties that allow them to be used as effective meliorants;
– favorable granulometric composition of glauconite sands reduces the cost of preliminary preparation of raw materials;
– glauconite sands exhibit pronounced ion-exchange properties and are effective sorbents, which makes it possible to reduce the ecotoxic properties of soils and ensure high quality and yield of cultivated crops;
– glauconites are able to actively sorb and bind water, which prevents the caking of ammonium nitrate;
– Kashkadarya region has large reserves of this raw material. According to experts, the annual reserve in this deposit is 1.2 million tons of glauconite sands that do not find practical application.
At present, there are convincing data confirming the expediency of using glauconites in agricultural practice. It has been established that the introduction of glauconite sands in single doses of 15-20 t/ha leads to an increase in the content of available forms of potassium, phosphorus and sulfur in the soil, which contributes to an increase in crop yields.
It should be noted that the composition and properties of glauconite sands of various deposits are characterized by high variability. It is known that the content of potassium oxide (6-7%), phosphorus oxide (up to 3%) in the glauconite sands of the Mabikansky glauconite mine. The color is dark gray, almost black.
The experimental part. Glauconite sands of the Mabikan glauconite mine (Dehkanabad district of Kashkadarya region) and granular ammonium nitrate produced by JSC “Farg’onaazot” were used to perform experimental studies. The content of mobile phosphorus in glauconite sands was 6.1 mg/dm3.
The grinding and mixing of the components was carried out manually. The composition of the mixture was calculated in such a way that the nitrogen content in the finished dried granules was 27-28%.
For granulation, a paste consisting of ammonium nitrate sifted through a mesh (0.25×0.25 m) of glauconite sand and water was used.
The mixture was heated to 135 ° C and passed through an oscillating sieve with 2×2 mm cells so that the granules fell from a height of 1 m onto the pallet in which the rolling process was carried out. The rolled pellets were placed in a drying cabinet to remove moisture to a residual humidity of no more than 1%.
The mass fraction of ammonium nitrate and nitrogen in the finished granules was determined according to GOST 14839.3-69.
The strength of the pellets was measured in kg per pellet. The pellet was installed between two ceramic tiles and a load of various weights was placed on the top tile. The weight of the cargo was recorded, at which the destruction of fertilizer granules occurred.
During the experiment, it was found that glauconite sand is easily mixed with ammonium nitrate to form a paste. To prepare a stable composition suitable for forming granules, it is necessary to strictly control the humidity and temperature of the composition.
Stabilization (drying) of granules should be carried out at relatively low temperatures. An increase in temperature to 100 ° C leads to a violation of the uniformity of the granules, since the surface of the granules is covered with white crystals of ammonium nitrate. To increase the speed of drying granules, you can use the method of active ventilation of the drying chamber.
The granules obtained during the experiment have a dark gray color and can withstand a load of at least 2 kg per granule
When the granules get into the water, they spontaneously crumble within 1-2 minutes, while ammonium nitrate goes into solution, and the auxiliary component (glauconite sand) remains in the sediment.
Conclusions. Ammonium nitrate granules with the addition of glauconite sand do not show signs of caking or destruction when stored in an open form indoors for 6 months. Granules of pure ammonium nitrate in similar conditions begin to collapse and clump.
The performed studies allow us to conclude that the glauconite sands of the Mabikan glauconite mine (Dehkanabad district of Kashkadarya region), containing up to 30% glauconite, can be used as an additive to improve the properties of ammonium nitrate.
LIST OF SOURCES USED
1. Чернышев А.К., Левин Б.В., Туголуков А.В., Огарков А.А., Ильин В.А. Аммиачная селитра: свойства, производство, применение. – М.: ЗАО «ИНФОХИМ», 2009. – 544 с.
2. Технологический регламент производства аммиачной селитры (2012) ТР КазАзот 10.53.1011.004-12.
3. ГОСТ 2-2013. Селитра аммиачная. Технические условия. – М.: Стандартинформ, 2014. – 65 с.
4. Справочник азотчика / под ред. Е.Я. Мельникова. Изд. – 2-е, перер. – М.: Химия, 1987. – 464 с.
5. Позин М.Е. Технология минеральных удобрений: учебник для вузов. – 6-е изд., перераб. – Л.: Химия, 1989. – 352 с.
6. Romania Z. T., Larisa A.K., Alexandru O. Ammonium nitrate explosions. Case study: the Mihăilesti accident // ECOTERRA – Journal of Environmental Research and Protection. – 2015. – Vol.12, №2. – Р.56-60.
7. Dechy N., Bourdeaux T., Ayrault N., Kordek M., Le Coze J. First lessons of the Toulouse ammonium nitrate disaster, 21 September 2001, AZF plant, France, //
J. Hazard Mater. – 2004. – Vol. 111, №1-3. – Р.131-138.
8. Попок В.Н., Бычин Н.В., Попок Н.И. Характеристики полиморфных модификаций промышленных марок и фазостабилизированного нитрата аммония // Боеприпасы и высокоэнергетические конденсированные системы. -2009. – № 1. – С. 20-26.
9. Vargeese A.A., Satyawati S.J., Krishnamurthy V.N. Effect of method of crystallization on the IV-III and IV-II polymorphic transitions of ammonium nitrate //Journal of Hazardous Materials. – 2009. -№161.- Р. 373-379.
10. Нечипоренко Г.Н., Головина Н.И., Шилов Г.В. Применение сокристаллизатов нитрата аммония как способ устранения фазовых переходов в кристаллической решетке нитрата аммония // HEMs-2004: Сб. докл. междунар. конф. – Белокуриха – Бийск: ФГУП «ФНПЦ «Алтай», 2004. – С. 16-19. st
11. Головина Н.И., Манелис Г.Б., Лемперт Д.Б. Нечипоренко Г.Н., Долганова Г.П., Немцев Г.Г. Кинетика прямого и обратного фазовых переходов IV-III в кристаллической решетке нитрата аммония // HEMs-2004: Сб. докл. междунар. конф. – Белокуриха – Бийск: ФГУП «ФНПЦ «Алтай», 2004. – С. 3740.
12. Кулацкий Н.С., Кряжева М.В., Противень И.Н., Савенков А.С. Исследование кинетики модификационного превращения IV-III нитрата аммония с добавкой сульфата кальция // Вопросы химии и химической технологии. – 2004. – № 4. – С. 172-174.
13. Клякин Г.Ф., Таранушич В.А. Модифицирование аммиачной селитры добавкой нитрата калия // Сб. тр. Общеросс. науч. – технич. конф. «Новые технологии в азотной промышленности». Ставрополь: СевКавГТУ, 2007. – С.
67-69.
14. Киселев С. H., Никифоров А. Е., Седова О.А. Влияние размера частиц на полиморфные переходы аммиачной селитры // Вестник Казанского технологического университета. – 2001. – № 2. – C. 120-124.
15. Кодиров Б.Х., Хамрокулов З.А. Крупнейшие пожары и взрывы аммиачной селитры, произошедшие в истории человечества// Экспериментал тадқиқотлар №1 (2023) Б. 7-11.
16. Kodirov, B., Sadiyeva, N., Isgenderova, S., Cherepnova, Y., Afandiyeva, L., Quliyeva, E., … & Shaumarov, A. CHEMICAL SCIENCES.
17. Bekzod Khomidzhonovich Kodirov. The largest explosions of ammonium nitrate in the XXI century. Colloquium-journal (ISSN 2520-2480), №1 (124), 50-55.
18. Никифоров А.Е. Пути фазовой стабилизации нитрата аммония // Современные проблемы технической химии: матер. докладов междунар. науч. – техн. и методич. конф. – Казань, 2004. – С. 442-443.
19. Пат. 2182143 Российская Федерация, МПК 7 С05С1/02, C05G 1/08. Способ получения стабилизированной аммиачной селитры / Наумов С. М., Коряков В.В., Кузнецов А.Г., Полякова О.А., Соловьев Б.А., Гарин Ю.М.; заявитель и патентообладатель АО “Минудобрения”. – №2000123962/12; заявл. 18.09.2000; опубл. 10.05.2002.
20. Пат. 7147830 Соединённые Штаты Америки, МПК 7 С 01 С 1/18. Стабилизированные гранулы нитрата аммония / Stabilized ammonium nitrate
granules / Kemira Growhow Oyj, Hero Heikki, Poukari Juhani. Jianzhou Wu; заявитель и патентообладатель Kemira Growhow Oyj (Finland). – № 11/206875;
заявл. 19.08.05; опубл. 12.12.06.
21. Колесников В.П., Москаленко Л.В. Термографические исследования модификационных превращений удобрения, полученного на основе аммиачной селитры // Химическая промышленность сегодня. – 2006 – № 7. – С. 18-21.
22. Brockel U., Wahl M., Kirsch R., Feise H.J. Formation and growth of crystal bridges in bulk solids // Chem. Eng. Technol. – 2006. – Vol. 29, № 6. – Р. 691-695.
23. Komunjer L., Affolter C. Absorption – evaporation kinetics of water vapour
on highly hygroscopic powder: case of ammonium nitrate // Powder Technol. – 2005. Vol. 157, №1. – Р. 67-71.
24. ГОСТ 21560.2-82 Удобрения минеральные. Метод определения статической прочности гранул. – ИУС.: – 2003 – С. 18-21.
25. Петропавловский И.А., Андриянова Е.А., Соколов В.В., Почиталкина И.А. Определение статической прочности гранул минеральных удобрений // Мир серы, N, P и K. – 2012. – Т. 6, – С. 8-13.
26. Kodirov B.X., Tojiyev R.R. Nitrat ammoniy suyuqlanmasiga glaukonit mineralini qo’shish asosida mikroelementlarga boyitilgan kompleks azotli mineral o’g’it olish. Scientific-technical journal (STJ FerPI, ФарПИ ИТЖ, НТЖ ФерПИ, 2022, Т.26.спец.выпуск №9), 195-198.
27. Колесников В.П., Москаленко Л.В., Белоусова Ю.Е. Влияние инертной добавки (цеолита) на прочность аммиачной селитры // Сб. тр. Общеросс. науч. – технич. конф. «Новые технологии в азотной промышленности». Ставрополь: СевКавГТУ, 2003. – С. 26-29.
28. Намазов Ш.С., Курбаниязов Р.К., Реймов А.М., Беглов Б.М. Прочность
гранул аммиачной селитры с добавками фосфоритов Центральных Кызылкумов // Химическая промышленность. – 2008. – Т.85, №2. – С. 65-70.
29. Leaper M.C., Bradley M.S., Cleaver J.A.S., Bridle I., Reed A.R., AbouChakra
H., Tüzün U. Constructing an engineering model for moisture migration in bulk solids as a prelude to predicting moisture migration caking // Adv. Powder Technol. – 2002. – Vol. 13, №4. – Р. 411-424.
30. 31 Таран Ю.А., Тaран А.В. Основные азотосодержащие минеральные
удобрения и технические решения для улучшения их качества // Изв. вузов.
Химия и хим. технол. – 2016. Т. 59, – №3. – С. 49-54.
31. Ботиров Б.Б., Беглов Б.М. Пути повышения качества аммиачной селитры // Хим. технол. Контроль и упр. – 2008. – №6. – С. 12-24.
32. Шведов К.К., Рубцов Ю.И. Оценка безопасности технологии получения
гранулирования и хранения аммиачной селитры // докл. на науч. – практ. конф «Современное состояние и проблемы производства аммиачной селитры»: ЗАО «Инфохим». – Спецвыпуск. – 2004. – С. 39-44.
33. Конвисар Л.В., Мошкович Е.Б. К вопросу обеспечения безопасной работы производства аммиачной селитры // Химическая промышленность. – 2002. – №6. – С. 40-42.
34. Жмай Л., Христианова Е. Аммиачная селитра в России и в мире. Современная ситуация и перспективы // Мир серы, N, P и K. – 2004, № 2. – С. 812.
35. Щеголев О.А. Состояние и проблемы производства аммиачной селитры //
Мир серы, N, Р и К. – 2004, – №2. – С.1-6.
36. Пат. 2416570 Российская Федерация, МПК С05С 1/18 (2006.01). Способ
получения водоустойчивого нитрата аммония (аммиачной селитры) / Михайдов Ю.М., Гатина Р.Ф., Хацринов А.И., Батырев А.В., Меркин А.А., Федотов П.И.; заявитель и патентообладатель Федеральное казенное предприятие «Государственный научно-исследовательский институт химических продуктов». – № 200912598/05; заявл. 06.07.2009; опубл. 20.04.2011.
37. Пат. 2480411 Российская Федерация, МПК С05С 1/18 (2006.01). Способ
получения водоустойчивого нитрата аммония / Михайлов Ю.М., Гатина Р.Ф.,
Хацринов А.И., Климович О.В.; заявитель и патентообладатель Михайлов
Ю.М., Гатина Р.Ф. – № 2010128039/05; заявл. 06.07. 2010; опубл. 27.04.2013.
38. Щеголев О.А. Состояние и проблемы производства аммиачной селитры
// Мир серы, N, Р и К. – 2004, – № 2. – С. 1-6.
39. Турдиалиев У.М., Намазов Ш.С., Реймов А.М., Сейтназаров А.Р., Беглов Б.М. Неслеживающаяся аммиачная селитра с добавкой бентонитовой глины Каттакурганского месторождения // Химический журнал Казахстана. – 2016. – №1. – С. 390-404.
40 Пат. 2491261 Российская Федерация, МПК С05С 1/00 (2006.01). Способ
получения неслеживающейся аммиачной селитры / Мухачева Т.Е., Медянцева Д.Г., Захарова О.М., Гуськова М.В., Зубарева Л.В.; заявитель и
патентообладатель ОАО «ЗМУ» Кирово-Чепецкого химического комбината. – №2012107181/13; заявл. 27.02.2012; опубл. 27.08.2013.
41. Kirilov PI. J. Non-caking fertilizers from ammonium nitrate and supplementary nutrients // Univ. Chem. Technol. And Met. – 2005. Vol. 40, №3. – Р. 209-212.
42. Нагалешкин Д.А., Гришаев И.Г., Долгов В.В. Исследование гигроскопичности смесей нитрата и сульфата аммония // Хим. технол. – 2011. -№10. – С. 593-597.
43. Kydyralieva A.D., Besterekov U.B., Petropavlovskiy I.A., Yermekov S.R. Thermal decomposition of ammonium nitrate: кinetics of the processes // V International Conference “Industrial Technologies and Engineering” ICITE – 2018. – Vol. 1. – Shimkent, 2018. – P. 212–216.
44. Махоткин И. А., Сахаров И. Ю., Махоткин А. Ф., Сахаров Ю. Н. Закономерности кинетики абсорбции аммиака азотной кислотой в условиях
производства аммиачной селитры // Вестн. Казанского техн. у-та. -2013. -Т. 16, №14. – С. 74 – 75.
45. Лавров В.В., Шведов К.К. О взрывоопасности аммиачной селитры и удобрений на ее основе // Научно-технические новости: ЗАО «ИНФОХИМ». – Спецвыпуск. – 2004. – №4. – С. 44-49.
46. Shen L., Wang X. Thermal stability assessment of antiexplosive ammonium
nitrate // Journal Univ. Sci. and Technol. Beijing. – 2005. – Vol. 1, №12. – Р. 12-15.
47. Соснии В.Г. Исследование взрывоопасности аммиачной селитры // докл. науч. – практ. конф. «Современное состояние и проблемы производства аммиачной селитры».: ЗАО «ИНФОХИМ». – Спецвыпуск. – 2004. -№2. – С. 5658.
48. Oxley J.C., Smith J.L., Rogers E., Yu M. Ammonium nitrate: thermal stability and explosivity modifiers //Thermochim. Acta. -2002.-Vol.384,№1-2. -Р.23-45.
49. Kydyralieva A.D., Besterekov U.B., Petropavlovskiy I.A., Nazarbeк U.B., Bolysbek A.A. Thermal decomposition of ammonia saltpeter: the process kinetics
and balance, mass calculations // V International Conference “Industrial Technologies and Engineering” ICITE – 2018. – Vol. 1. – Shуmkent, 2018. – P. 221226.
50. Besterekov U., Kydyralieva A.D., et al. Mass balance calculations of processes of ammonia saltpeter thermal decomposition and nitric acid absorption of ammonia // Bulletin of the Karaganda University. “Chemistry” series. – 2019. – Vol. 96. – P. 92-97. doi:10.31489/2019ch4/92-97.
51. Рубцов Ю.И., Казанов А.И. Скорость термического разложения твердого нитрата аммония в присутствии влаги и избытка азотной кислоты //Журнал прикладной химии. – 1990. – Т. 60. – №1. -С. 3-7.
52. Han Zh., Sachdeva S., Papadaki M.I., Mannan S. Effects of inhibitor and promoter mixtures on ammonium nitrate fertilizer explosion hazards // Thermochim. Acta. – 2016. – Vol. 624. – P. 69-75.
53. Tang Sh., Liu Z., Zhu G., Tong X., Lu Ch. Effect of additives on detonation
safety and heat stability of ammonium nitrate // Huafei Gongye. – 2003. – Vol. 30, № 4. – P. 28-32.
54. Buczkowski, D., Zygmunt, В., Pagowski, W. Effect of addition of inorganic substances on reduction of the explosibility of ammonium nitrate // Biuletyn Wojskowej Akademii Technicznej. – 2004. – Vol. 53, № 2-3. – P. 95-107.
55. Sun Jinhua. Catalytic effects of inorganic acids on the decomposition of ammonium nitrate / Sun Jinhua, Sun Zhanhui, Wang Qing-song, Ding Hui, Wang
Tong, Jiang Chuansheng // Journal Hazardous Materials. -2005.-№ 1-3 (127).-C. 204210.
56. Волкова А.В. Рынок минеральных удобрений – 2019 / Национальный исследовательский университет Высшая школа экономики Центр развития. –
2019. – 52 c.
57. РБК. Исследование рынков. Мировой и российский рынок минеральных
удобрений // http://marketing.rbc.ru
58. https://www.mordorintelligence.com/ru/industry-reports/ammonium-nitrate-market
