I NEVER THINK ABOUT YOU WHEN IT RAINS ANYMORE I never think about you when it rains anymore except for tonight when I am for some reason. It could be the way the air smells a little like mold, only it smells good, not bad and it reminds me of some other time but I don’t remember when. I hope next time it rains I’ll continue my process of forgetting all about you more and more often but the rain has a way of getting in when it gets to falling heavy. I don’t know. I breathed you in for so long and it’s been years since then but I know my body hasn’t expelled all of you yet. I never think about you when it rains anymore except for tonight when I am for some reason. Water is getting in through the one window I’ve left open, over in the corner. I’ll get up and close it but not yet. Eventually. Not yet. THE RIVERS The river of your spine, the soft and gentle slopes of your body. The deep well of your belly, its rich sediment; the two burning coals that were your eyes, moistening and filling the room with steam. Your mouth when I was hungry; its dewy texture, its ripe flavor. Your breasts a cottony riverside when I needed to rest and bathe and drink. My hair damp with the evaluation of your flesh, my bare feet leaving wet half-prints on the floor beside the bed. Your thighs two more rivers flowing up and down and me swimming all along them a long time ago before this now-dusty valley, abandoned and long weary of metaphors, went dry. THERE ARE PEOPLE There are people who sit alone drinking coffee and they listen to every gulp as it falls down their throats and vibrates in their ears. There are people who smoke cigarettes and they hold them in a certain effete way, watching each puff of smoke as it emanates from their browning lips and rises up the room like a mist of vines. There are people who are content to eat alone in a brightly lit restaurant reading something on their phone while they eat french fries without looking at them. There are people who don’t notice when someone has entered the room and there are people who compliment anything that they secretly find unattractive or vile. There are people who drink and people who don’t drink anymore and people who have never swallowed even a single drop. There are people who think they love God and people who curse at the mention of His name and people who don’t believe he exists at all and there are people like us who don’t pretend we know anything about anything. THE TOMBOY She only lived around the block from us for a summer or so and I can’t remember her name but I can close my eyes now and see her as clearly as I could when we were ten years old and she played Army with us. She had short brown hair a little darker than mine and just as messily arranged on her head and she could and would do all the things a boy her age did. She played hockey and baseball with us and I had this enormous crush on her even though she dressed and acted and kind-of looked a bit like a boy. Never did I say anything or do anything about it, of course. I was ten. I kept everything to myself like most of the kids did. I tried to be on her team (or side when it came to Army) whenever she came out to play with us and no matter how fast she could run, how far she could throw or how well she could imitate the sound of a machine gun, she was still a girl to me. She had eyes like a girl. No boy’s eyes would ever make me feel like that. Her sweat smelled different than my sweat and when it sat in beads on her neck as she stood with hands on knees at second base with eyes squinting in the sun I knew that she was a girl and that I liked girls – especially her. She spat on the ground and scratched her short boy’s haircut while I snuck my glances, feeling many things – none of them confusion. YOUR DUSKY STEM! Your dusky stem! Your bright brilliant husk! Watching you bloom at night, My lovely evening primrose, Your petal soul so yellow, So delicate to touch, So indestructible in the wind That never stops blowing. You bring me your medicine And your certain loveliness Each evening that you open For me, just for me, only me. You black-eyed sorceress With your thighs that are Held by roots that love the earth. Your blatant purple stigma! Your anthers that shine! Your filaments glistening with new dew! Your sheltered husk that hides The seeds and the fruit That nourish me And your sepals that hold such beauty With an animal’s natural grace. You black-eyed mistress With your legs that shake But do not bend, Held by roots that love the earth.
Category Archives: CHAOS
Synchronized Chaos Mid-November: The World That Dwarfs and Outlasts Us
We continue to express sorrow over what’s happening in so many different parts of the world and encourage our readers to support people and the planet.

Also, we are hosting our Metamorphosis gathering again! This is a chance for people to share music, art, and writing and to dialogue across different generations (hence the name, the concept of ideas morphing and changing over the years). So far photographer Rebecca Kelly and English/Spanish bilingual poet Bridgett Rex are part of the lineup and more are welcome! This event is also a benefit for the grassroots Afghan women-led group RAWA, which is currently supporting educational and income generation and literacy projects in Afghanistan as well as assisting earthquake survivors. (We don’t charge or process the cash, you are free to donate online on your own and then attend!)
This will be Sunday, December 31st, 2-4 pm in the fellowship hall of Davis Lutheran Church at 317 East 8th Street in Davis, California. It’s a nonreligious event open to all, the church has graciously allowed us to use the meeting room.
You may sign up here for event reminders. RSVP appreciated but not required.
This issue draws us into a full sensory experience, surrounding us with places and worlds larger and more vast than ourselves.
Vernon Frazer’s pieces rumble with a smorgasbord of rhythmic and clanging instruments and sounds while Joshua Martin sends up a plethora of sonic syllables. Mahbub Alam stares and contemplates the beauty of nature and the Taj Mahal. Christina Poythress highlights through tactile details the rich nightlife within the world’s soil. Kathleen Hulser draws on mathematical concepts as metaphors for how life changes affect and circumscribe our lives.

Jim Meirose illuminates the sensory experience of playing outside on the grass on a nice sunny day while Lorraine Caputo wanders off trail in South America: evenings, out-of-the-way streets, and less crowded areas.
Rafiul Islam speculates on inter-planetary relations in a society where multiple sentient species inhabit multiple planets.
Bekzod Quodirov outlines ways to make ammonium nitrate safer and more stable as a fertilizer and an industrial tool.

Uruguayan countryside, fisherman, photo c/o Juan Carlos Gonzalez
Even our own, more human-scale worlds contain more detail that we often grasp at first glance.
Sophia Fastaia remembers the joy, wonder, comfort and danger of childhood, all in one birthday party.
Chloe Schoenfeld’s piece probes opposites and finding and befriending one’s shadow self. Pascal Lockwood-Villa surveys a vacation in the tropics through the lens of photos that reflect different dimensions of human nature.
Susan Hodara details the common sensory experience of drying off after a shower while J.D. Nelson observes daily life and snacks within a homeless shelter.
Philip Butera describes with sensory details the underside of a circus after the show, referencing the work of repackaging the illusion.
Duane Vorhees’ work explores coupling and fertility from several big-picture spiritual and grounded, natural angles. Aklima Ankhi describes the search for an intense emotional connection with a lover that goes beyond the fleeting happiness of the everyday.
Slavica Pejovic ponders love, closeness, completeness, and connection. Aasma Tahir rhapsodizes about the subconscious worlds of nighttime, romance, and the imagination. Kristy Ann Raines describes the intense emotional experiences of love lost and regained.

While our universe can be glorious, it can also be tragic, with forces beyond our control.
Ari Nystrom-Rice reflects on the fragility of his knowledge and sense of place in his world through the metaphor of a child’s toy boat exposed to the elements.
Nilufar Ergasheva illustrates the dangers of the winter season in rural villages, with cold and wild animals on the prowl, while Christopher Bernard renders appendicitis and surgery into poetry.
Mykyta Ryzhykh probes where we can find meaning and tenderness in a war-ravaged world where death seems frequent and life seems meaningless. Atagulla Satbaev shares how we delude ourselves into thinking love is eternal: time and death separate everyone. Michael Lee Johnson reflects on his own mortality and attempts to find eternal love in living death, rather than in the capriciousness of life.
Graciela Noemi Villaverde’s piece renders grief into somnambulant surrealism, a panoply of dream images while Alden Joe evokes the pain of lost love with imagery of tigers and predation. Suleiman Gado Mansir sends up a surreal dream sequence illustrating how our minds attempt to process the world’s violence.

Sometimes, we wonder what place we have in such a large world. Will the universe overwhelm and consume us?
Alma Ryan explores the season of fall with a meditation on falling, death, and the ways we let ourselves go. J.J. Campbell’s work turns solemn this month as he ponders various kinds of death and forms of passing away.
Zahro Shamsiyya reflects on the brevity of life and the need to savor the experience. Jerry Langdon reflects on the changing of seasons and the passing of a friend.
Gabriel Flores Benard shows the tragic ways continued abuse can shape a still-forming personality.
Even apart from mortality and injustice, everyday human psychology can be a mysterious and unmapped landscape.

Zosia Mosur illustrates how we sculpt and train and also harm and punish our physical selves.
Taylor Dibbert’s speaker speculates on what his midlife decades will bring, while Noah Berlatsky highlights the common human experience of procrastination and Shirley Smothers relates her efforts to maintain inner peace.
Shamsiya Khudoynazarova Turumovna laments that real life can’t be like the novels she reads. Azemina Krehic compares herself to a linden tree and wishes she possessed its strength, but finds herself instead in the tree’s biological complexity.
Yet, we as humans do not have to be passive in the face of such a large and grand universe. There are roles we can play, even as individuals, that allow us selfhood and transcendence.
Diyora Abdujabborova’s reflects on the value of women’s leadership and nurturing roles in Uzbek society. Anila Bukhari speaks to the earnest desire of girls living in poverty to get an education.

Christina Chin and Uchechukwu Onyedikam collaborate on haikus that are translated into English, Taiwanese, and Igbo and highlight moments of people collaborating with nature. Nery Santos Gomez illustrates the joy she takes moving in unison while riding a beloved horse.
Daniel De Culla’s photography focuses on low-key ways we alter or adjust our environment: clothes, sketches, bushes we plant. Isabel Gomez de Diego illustrates moments where nature (small children and plants) integrates into our built environments.
Sayedur Rahman demonstrates the resilience and strength of refugees creating new lives in their new homelands. Jacques Fleury asserts his place in the world as a Black man, self confident even in spaces not created with him in mind.
Christina Chin and Paul Callus also collaborate on further haikus translated into English, Mandarin and Maltese that celebrate the mastery of crafts: cooking and painting.
Annie Johnson speaks to the transcendent immortality she finds through stepping out of herself to create art that will outlast her.
Mark Young reflects on the values and accomplishments of his Boomer generation in terms of shaping society while questioning the uses of similar government power today.
Z.I. Mahmud outlines Jane Eyre’s character growth and self-assertion in Charlotte Bronte’s novel while Shokirova Zarnigor Shuhratjanovna urges patience for people seeking the meaning of their lives.

Orzigul Sherova shares how she learned to draw on her fantasies as an inspiration rather than as a way to avoid achieving her real-world goals.
In Nahyean Bin Khalid’s take on a haunted mansion horror tale, his protagonist frees undead souls trapped in the home, but stays to become their caretaker rather than escaping, getting killed, or kicking the ghosts out.
Maja Milojkovic’s piece encourages us to heal and move forward from grief. Nilufar Rukhillayeva’s translation of Erkin Vahidov’s Uzbek poem points to a larger societal step forward, the passage of time and renewal that comes with the New Year.
Jaylan Salah reviews Daniel Radcliffe’s new HBO show The Boy who Lived, about David Holmes, his stunt double who became paralyzed after an injury on set and who worked with quiet courage and dignity to rebuild his life.
Even if our places in the universe are relatively small in the grand scheme of things, it matters how we fill our places because our behavior and choices affect those around us.

Rasheed Olayemi’s poem demonstrates how corruption at both individual and governmental levels weakens a country’s economy.
Daniel De Culla calls out the hypocrisy of people who focus more on looking good at charity balls rather than helping others, especially in wartime.
Mesfakus Salahin’s narrators are wise beyond their years in terms of their ability to love and respect and connect with other people. Salahin urges adult world leaders to hold to that level of maturity.
Elmaya Jabbarova urges the world to wake up and turn back towards life and justice.
Lilian Dipasupil Kunimasa fondly remembers her low-tech but fun childhood visits to her grandparents’ country town, and urges compassion for those with HIV/AIDS.
Family, culture, love, and heritage can be vital to grounding us and giving us the strength to withstand a rough universe.

Aziza Gayratova expresses respect for her parents and the strength family love gives her to endure life’s injustices.
Wazed Abdullah reminds us of how essential love and caring is to life while Faleeha Hassan speaks to a mother’s wish to protect her son during wartime in her poem, translated by William Hutchins.
Shahnoza Ochildiyeva offers up a colorful paean to her native Uzbekistan while Yahya Azeroglu pays tribute to Ataturk, the founder of modern Turkey.
Fahim relates a story of courage and loyalty among Bangladeshi soldiers at the country’s founding.
Finally, to come back to nature and the vast universe outside of our own species, Brian Barbeito reflects on the wisdom of nature to outlast humanity. He also considers how mysterious the sea remains, even after millennia of sailing.
Poetry from Erkin Vahidov, translated from Uzbek to English by Nilufar Rukhillayeva

New Year poem The human verb is surprised by surprise, There is no world without criteria. By measuring the stars, It also touches infinity. He is the owner, he is the slave forever To the beliefs he found, Don't say, even eterned, To the moments that happened. Collect hours from minutes, The days make up the months, He does not cry that life has passed, He celebrates the end of the year. Even though the wrinkles are increasing, As the years go by, Rejoice - more children, He rejoices - his age grows. It is a dream of endless There is a basis for hopeful faith: Man is immortal. Little by little It just goes to eternity. Erkin Vahidov Translator: Nilufar Rukhillayeva(1st year student of the Faculty of Foreign Philology of the National University of Uzbekistan named after Mirzo Ulugbek)

Poetry from Mashhura Usmonova

Great birds
In the search of warm places,
Where are you going again?
Oh, cranes please get back,
And build the home of affection in heart.
Do not be afraid from the first fallen leaf,
Do not fly away, great birds.
Want to see a familiar face,
Birds like me whose hearts are burnt.
I follow you from behind,
All of you are leaving happily.
But remember I will wait,
Wait for you to come to me.
But I got upset from you, I have to say,
Listen to me hey, cranes.
One of your friends that has fallen from the row,
Is struggling, do not you see?!
And you, you are being just reckless,
Maybe it had the same intention as you.
The injured bird kept looking,
Looking long, from your back.
Oh great birds, great birds,
Burnt- heart- birds just like me.
Despite being unfaithful like humans,
Still, everyone loves you all.
Mashhhura Usmonova Zafarjon’s daughter was born on May 16, 2006 in Gallaorol district, Jizzakh region the Republic of Uzbekistan. Currently 16 years old. She has been practicing writing poetry since her 10 years old. Now, she is author of about 100 poems. She is member of the international organizations Egypt, Indonesia, Pakistan, Argentina and India. In 2019, the author’s book of poetry entitled “Happy Childhood Message” was published. In 2022, the second author’s book of poetry entitled “Letter…” was put up for 26 countries under the Amazon online store of the United States. In addition, her works have been published in book collections of the United States of America, Turkey, Azerbaijan, Germany, Thailand, Canada, UK, Kenya and Moldova. She likes to read books and travel. Her future goal is to become a philologist.
Poetry from Shahnoza Ochildiyeva

Why I love my Motherland Why I love my Uzbekistan Let the wind tells about it. Take a deeper look at my motherland, It’s wearing a wreath from glorious history. Look for Ulegbek’s eyes there Who spend life staring at the stars in the sky In historical pages of my country All great beings are alive and alive. Maybe it is a childish love Let the world know its glory and fame. Namangan’s bright red apples And Samarkand’s baked shirmoy bread. I still have a smell in my throat Of sweet sumalak by my aunt Leads us right towards the dreams, A wish made in front of sumalak. Glittering on the nch of my country A bright necklace made of fame. Oh, friends, you will all see it too, Each necklace is a champion. Look at my lovely motherland It’s wearing an embroidered long dress Brightly different colours, Suits my beautiful country. Margilon satin is fine and tend The embroidery would adorn it fully Tying her waist with a decorative belt, Standing in body with full of beauty. Clothes worn by my Motherland Golden robe woven by Bukhara I think this is an another reason, Of the fiery and pure love of me. My country leads us in its hands, Thousands of kids, boys and girls. Towards a great bright future, All generations with enthusiasm. My country is lighting everywhere As a symbol of the brightest sun It’s dear to me as long as I live As my supposedly loving mother.
Ochildiyeva Shahnoza Abdivohid qizi was born on July 17, 2006 in the Republic of Uzbekistan, Surkhandarya region, Denov district. Presently, she studies at school number 49 in 10th grade.
Poetry from Aziza Gayratova
Parents My friend, listen to my words. Give thanks for every day. Bowing at the feet of your parents. Respect them and pray for them. May basil be scattered at your feet, mother. May your step be filled with light, May you have many happy days. Do not tire us by praying, mother. Dear Father, thank you You gave us a lot of mercy, You have blessed us no less than anyone else. We learned the science of goodness from you. How great is the word of parents, Appreciate them. You gave us your life, Give thanks to those who have parents
The world Forgetting the worries of the world a little I dream of the best days. I won't cry anymore, it's enough I look forward to my happy days To the tyranny of cruel people. I will not shed tears, I will be patient I don't yawn anymore, I don't care I live like an indifferent person O world, you have many oppressions. You took all my things. I will tell you about my pains. I will quietly laugh at you It's such a heavy unfaithful world I leave you my lions For the hearts that are close to me I leave loyalty and honesty.
Essay from Bekzod Qodirov
THE USE OF GLAUCONITE SANDS OF THE MABIKAN DEPOSIT TO IMPROVE THE PROPERTIES OF AMMONIUM NITRATE
KODIROV BEKZOD KHOMIDZHONOVICH, doctoral student
TOZHIEV RUSTAM RASULOVICH, Dean
FERGANA POLYTECHNIC INSTITUTE, UZBEKISTAN
e-mail: b.qodirov@ferpi.uz, Rustamjontojiyev658@gmail.com
Annotation. Ammonium nitrate is used both as a fertilizer in agriculture and as an ingredient for industrial explosives (for quarries, mine tunnels, etc.). The substance is stable under normal conditions of use, storage and transportation, but despite this stability, in the last century there were several accidents associated with a large number of ammonium nitrate, which has led to numerous deaths and injuries to people. This article analyzes in more detail the incidents related to the explosion of ammonium nitrate in the middle of the XX century (partially). Based on the official report on the conditions and consequences of the accident, various scenarios were considered using different TNT equivalence coefficients and the physical consequences of the explosion were determined.
Keywords: ammonium nitrate, properties, explosivity, hygroscopicity, porosity and particle density, qualitative indicators.
Introduction. Ammonium nitrate is an inorganic product of industrial synthesis, produced from gaseous ammonia and nitric acid. It was first obtained by Glauber in 1659. In its production form, it is a colorless crystalline substance with a content of 35% of total nitrogen in ammonia and nitrate forms. For fertilizing purposes, it is produced in granular form [1]. The physico-chemical properties of ammonium nitrate are regulated by the regulatory requirements of GOST 2-2013 [2,3].
Ammonium nitrate is well soluble in water. Its water solubility at 25oC exceeds 2 kg/kg of water, which is significantly higher than the solubility of many organic and inorganic compounds [4].
One of the important distinguishing characteristics of ammonium nitrate is its strong oxidizing ability in relation to a large number of inorganic and organic substances, which strongly affects its consumer and commodity properties. In recent years, explosions and fires caused by this characteristic of ammonium nitrate in a number of countries [5-7] have significantly reduced the demand for this important universal fertilizer products.
A characteristic specific indicator of ammonium nitrate is the polymorphism of its properties. This means its ability to change its crystal structure depending on the temperature of the medium. As is known [4,8-10], depending on temperature conditions, ammonium nitrate has five crystalline forms. At the same time, the structural transition in its composition from one crystal structure to another takes place under conditions of heat release or absorption, and leads to changes in the volume and density of crystals, as well as all its other physico-chemical properties. It has been established [11-14] that the III structural modification of ammonium nitrate is the most stable in relation to its transportation and storage in hot climatic conditions, a number of sufficiently effective ways of stabilizing its III structural modification have been found and developed [15-20].
Ammonium nitrate has a high traceability [3.21]. Because of this, in the practical application of ammonium nitrate as a fertilizer, there are significant losses of marketable products, additional costs are required when using the accumulated part of it [1,4]. As is known [4,22,23], the traceability of ammonium nitrate is caused by its high solubility, hygroscopicity, low strength of granules, as well as polymorphic transformations taking place in its structure. Therefore, the lower the solubility and hygroscopicity, the higher the strength of ammonium nitrate granules, the lower its traceability.
An important characteristic of the production ammonium nitrate is the mechanical strength of its granules. According to modern requirements [3], ammonium nitrate granules during transportation, storage and use should not be destroyed under mechanical loads. The strength of granules is characterized by three indicators: dynamic strength (Rd), abrasion resistance (At), static strength (Rd) [24,25]. The strength of ammonium nitrate granules is most significantly influenced by humidity, porosity and the size of the product granules [22,26,27]. With a decrease in humidity and the size of individual granules, as well as with an increase in their density, the strength of ammonium nitrate granules increases.
Ammonium nitrate, as a result of intensive moisture absorption, reduces its mechanical strength, collapses and cures [1,4, 28-30]. All this is due to its hygroscopicity. Because of this, the production ammonium nitrate needs to comply with special storage conditions, causes a number of difficulties in its practical use for fertilizing purposes [31-36].
Therefore, today everywhere both manufacturers of ammonium nitrate and its consumers are keenly interested in reducing its hygroscopicity by various methods and techniques [37-42].
The world practice of using ammonium nitrate indicates that it is characterized by flammability and explosiveness [45-48]. This is due to the fact that ammonium nitrate is prone to thermal decomposition and has strong oxidizing properties. Thermal decomposition of ammonium nitrate takes place with slow heating starting from 110oC and above. At low temperatures, the products of thermal decomposition are ammonia and nitric acid, and at high temperatures, nitrous oxide, nitrogen oxides and oxygen [4,49-51]. At the same time, in the high temperature region, the formation of the last products is explosive. Consequently, due to the thermal decomposition of ammonium nitrate, there is a loss of part of the product, as well as the practical use of ammonium nitrate becomes unsafe. Therefore, in order to completely suppress the process of thermal decomposition of ammonium nitrate or its effective deceleration, a number of methods and techniques for its stabilization are used in practice [52-55].
Ammonium nitrate is segmented by application (fertilizers, explosives and other applications), end users (agriculture, mining, defense and others) and geography (Asia-Pacific region, North America, Europe, South America, Middle East and Africa)). The report suggests the market size and forecasts of the ammonium nitrate market in volumes (kilotons) for all of the above segments.
The agricultural segment accounts for the main market share of ammonium nitrate.
In agriculture, fertilizers are used to enrich plant growth. They contain essential nutrients for plants that ensure healthy growth and protect them from diseases. There are various types of fertilizers that are available in soluble, dry crystalline forms and forms of prolonged action. Ammonium nitrate is one of such fertilizers, which is used to provide plants with a sufficient amount of nitrogen.
The ammonia fraction of ammonium nitrate is absorbed by the roots or gradually converted into nitrate by soil microorganisms. Many vegetable growers prefer an affordable nitrate plant food source and use ammonium nitrate. Livestock farmers like it for fertilizing pastures and hay, because it is less susceptible to evaporation losses than urea-based fertilizers when it remains on the soil surface.
Ammonium nitrate is usually mixed with other fertilizers, but these mixtures cannot be stored for a long time due to the tendency to absorb moisture from the air.
However, the explosion of 2,700 tons of ammonium nitrate in Beirut in 2020 demonstrates the importance of handling this substance and the need for stricter and clearly defined rules in the industry.
Consequently, due to the rapidly growing agricultural activity worldwide, the demand for solid ammonium nitrate is expected to grow rapidly during the forecast period (58).
Approximately half (50.5%) of the total global consumption of ammonium nitrate is accounted for by such major and developed countries of the world as the USA (17%), the Russian Federation (14%), France (5%), Brazil (2.5%), the UK (2%), and the rest is the share of all other countries of the world (Figure 1). Visual changes in the export and import of ammonium nitrate in physical terms are shown in Figure 2.
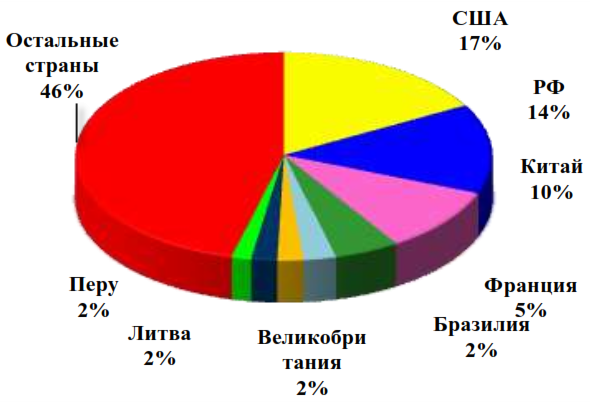
Source: IFA, Fertecon, company assessment [57]
Figure 1 – Structure of global consumption of ammonium nitrate in 2019 by leading countries of the world
In the CIS countries, the leaders in the market of nitrogen fertilizers, including ammonium nitrate, are the Russian Federation, Ukraine, Uzbekistan, Belarus and Kazakhstan, where today there are more than 28 modern production facilities [56,57].
There are currently 20 leading manufacturers and companies of ammonium nitrate in the world. Figure 2 shows that the Norwegian company “Yara” confidently leads in terms of production of ammonium nitrate, followed by three Russian companies “Uralchem”, “EuroChem”, “Akron” and Uzbek “Uzkimesanoat”.
Since 2008, restrictions on the supply of ammonium nitrate containing more than 28% nitrogen have been in effect in European countries. As a result, the export of domestic saltpeter to Europe, containing 34% nitrogen, was closed. In our country, certain measures are also being taken to restrict the export of ammonium nitrate to countries where military operations are underway or the domestic political situation is characterized by a high level of terrorist activity.
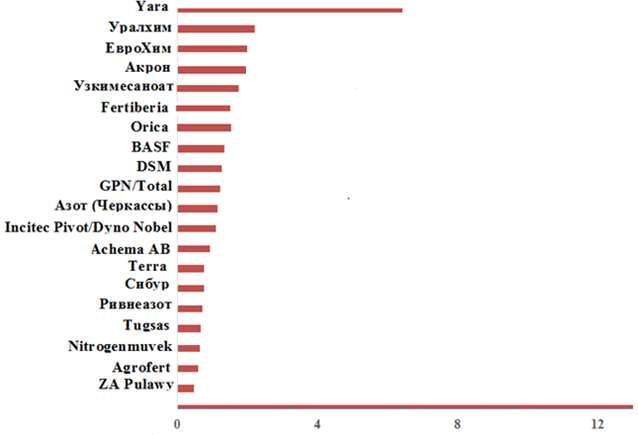
Source: based on the materials of the website www.uralchem.ru
Figure 2 – Dynamics of the volume of output of the sales market of 20 leading world manufacturers of ammonium nitrate, million tons
In this regard, the search for rational ways to reduce the explosive hazard of ammonium nitrate and increase its agrochemical characteristics is of great practical interest. The successful solution of these problems can help to increase the competitiveness of mineral fertilizers produced in our country and more actively promote them to the world market.
Currently, additives of limestone, chalk, chloride or potassium sulfate and some other mineral components are most often used to reduce the explosive hazard of ammonium nitrate (Kropacheva, RF).
The aim of the work was to study the possibility and feasibility of granulating ammonium nitrate in a mixture with glauconite sand from the Mabikan glauconite mine (Dehkanabad district of Kashkadarya region).
The choice of an additive is due to the following factors:
– glauconites are good sorbents capable of absorbing and retaining gases released during the decomposition of ammonium nitrate (reducing the threat of explosion);
– glauconite sands have a complex of valuable agrochemical properties that allow them to be used as effective meliorants;
– favorable granulometric composition of glauconite sands reduces the cost of preliminary preparation of raw materials;
– glauconite sands exhibit pronounced ion-exchange properties and are effective sorbents, which makes it possible to reduce the ecotoxic properties of soils and ensure high quality and yield of cultivated crops;
– glauconites are able to actively sorb and bind water, which prevents the caking of ammonium nitrate;
– Kashkadarya region has large reserves of this raw material. According to experts, the annual reserve in this deposit is 1.2 million tons of glauconite sands that do not find practical application.
At present, there are convincing data confirming the expediency of using glauconites in agricultural practice. It has been established that the introduction of glauconite sands in single doses of 15-20 t/ha leads to an increase in the content of available forms of potassium, phosphorus and sulfur in the soil, which contributes to an increase in crop yields.
It should be noted that the composition and properties of glauconite sands of various deposits are characterized by high variability. It is known that the content of potassium oxide (6-7%), phosphorus oxide (up to 3%) in the glauconite sands of the Mabikansky glauconite mine. The color is dark gray, almost black.
The experimental part. Glauconite sands of the Mabikan glauconite mine (Dehkanabad district of Kashkadarya region) and granular ammonium nitrate produced by JSC “Farg’onaazot” were used to perform experimental studies. The content of mobile phosphorus in glauconite sands was 6.1 mg/dm3.
The grinding and mixing of the components was carried out manually. The composition of the mixture was calculated in such a way that the nitrogen content in the finished dried granules was 27-28%.
For granulation, a paste consisting of ammonium nitrate sifted through a mesh (0.25×0.25 m) of glauconite sand and water was used.
The mixture was heated to 135 ° C and passed through an oscillating sieve with 2×2 mm cells so that the granules fell from a height of 1 m onto the pallet in which the rolling process was carried out. The rolled pellets were placed in a drying cabinet to remove moisture to a residual humidity of no more than 1%.
The mass fraction of ammonium nitrate and nitrogen in the finished granules was determined according to GOST 14839.3-69.
The strength of the pellets was measured in kg per pellet. The pellet was installed between two ceramic tiles and a load of various weights was placed on the top tile. The weight of the cargo was recorded, at which the destruction of fertilizer granules occurred.
During the experiment, it was found that glauconite sand is easily mixed with ammonium nitrate to form a paste. To prepare a stable composition suitable for forming granules, it is necessary to strictly control the humidity and temperature of the composition.
Stabilization (drying) of granules should be carried out at relatively low temperatures. An increase in temperature to 100 ° C leads to a violation of the uniformity of the granules, since the surface of the granules is covered with white crystals of ammonium nitrate. To increase the speed of drying granules, you can use the method of active ventilation of the drying chamber.
The granules obtained during the experiment have a dark gray color and can withstand a load of at least 2 kg per granule
When the granules get into the water, they spontaneously crumble within 1-2 minutes, while ammonium nitrate goes into solution, and the auxiliary component (glauconite sand) remains in the sediment.
Conclusions. Ammonium nitrate granules with the addition of glauconite sand do not show signs of caking or destruction when stored in an open form indoors for 6 months. Granules of pure ammonium nitrate in similar conditions begin to collapse and clump.
The performed studies allow us to conclude that the glauconite sands of the Mabikan glauconite mine (Dehkanabad district of Kashkadarya region), containing up to 30% glauconite, can be used as an additive to improve the properties of ammonium nitrate.
LIST OF SOURCES USED
1. Чернышев А.К., Левин Б.В., Туголуков А.В., Огарков А.А., Ильин В.А. Аммиачная селитра: свойства, производство, применение. – М.: ЗАО «ИНФОХИМ», 2009. – 544 с.
2. Технологический регламент производства аммиачной селитры (2012) ТР КазАзот 10.53.1011.004-12.
3. ГОСТ 2-2013. Селитра аммиачная. Технические условия. – М.: Стандартинформ, 2014. – 65 с.
4. Справочник азотчика / под ред. Е.Я. Мельникова. Изд. – 2-е, перер. – М.: Химия, 1987. – 464 с.
5. Позин М.Е. Технология минеральных удобрений: учебник для вузов. – 6-е изд., перераб. – Л.: Химия, 1989. – 352 с.
6. Romania Z. T., Larisa A.K., Alexandru O. Ammonium nitrate explosions. Case study: the Mihăilesti accident // ECOTERRA – Journal of Environmental Research and Protection. – 2015. – Vol.12, №2. – Р.56-60.
7. Dechy N., Bourdeaux T., Ayrault N., Kordek M., Le Coze J. First lessons of the Toulouse ammonium nitrate disaster, 21 September 2001, AZF plant, France, //
J. Hazard Mater. – 2004. – Vol. 111, №1-3. – Р.131-138.
8. Попок В.Н., Бычин Н.В., Попок Н.И. Характеристики полиморфных модификаций промышленных марок и фазостабилизированного нитрата аммония // Боеприпасы и высокоэнергетические конденсированные системы. -2009. – № 1. – С. 20-26.
9. Vargeese A.A., Satyawati S.J., Krishnamurthy V.N. Effect of method of crystallization on the IV-III and IV-II polymorphic transitions of ammonium nitrate //Journal of Hazardous Materials. – 2009. -№161.- Р. 373-379.
10. Нечипоренко Г.Н., Головина Н.И., Шилов Г.В. Применение сокристаллизатов нитрата аммония как способ устранения фазовых переходов в кристаллической решетке нитрата аммония // HEMs-2004: Сб. докл. междунар. конф. – Белокуриха – Бийск: ФГУП «ФНПЦ «Алтай», 2004. – С. 16-19. st
11. Головина Н.И., Манелис Г.Б., Лемперт Д.Б. Нечипоренко Г.Н., Долганова Г.П., Немцев Г.Г. Кинетика прямого и обратного фазовых переходов IV-III в кристаллической решетке нитрата аммония // HEMs-2004: Сб. докл. междунар. конф. – Белокуриха – Бийск: ФГУП «ФНПЦ «Алтай», 2004. – С. 3740.
12. Кулацкий Н.С., Кряжева М.В., Противень И.Н., Савенков А.С. Исследование кинетики модификационного превращения IV-III нитрата аммония с добавкой сульфата кальция // Вопросы химии и химической технологии. – 2004. – № 4. – С. 172-174.
13. Клякин Г.Ф., Таранушич В.А. Модифицирование аммиачной селитры добавкой нитрата калия // Сб. тр. Общеросс. науч. – технич. конф. «Новые технологии в азотной промышленности». Ставрополь: СевКавГТУ, 2007. – С.
67-69.
14. Киселев С. H., Никифоров А. Е., Седова О.А. Влияние размера частиц на полиморфные переходы аммиачной селитры // Вестник Казанского технологического университета. – 2001. – № 2. – C. 120-124.
15. Кодиров Б.Х., Хамрокулов З.А. Крупнейшие пожары и взрывы аммиачной селитры, произошедшие в истории человечества// Экспериментал тадқиқотлар №1 (2023) Б. 7-11.
16. Kodirov, B., Sadiyeva, N., Isgenderova, S., Cherepnova, Y., Afandiyeva, L., Quliyeva, E., … & Shaumarov, A. CHEMICAL SCIENCES.
17. Bekzod Khomidzhonovich Kodirov. The largest explosions of ammonium nitrate in the XXI century. Colloquium-journal (ISSN 2520-2480), №1 (124), 50-55.
18. Никифоров А.Е. Пути фазовой стабилизации нитрата аммония // Современные проблемы технической химии: матер. докладов междунар. науч. – техн. и методич. конф. – Казань, 2004. – С. 442-443.
19. Пат. 2182143 Российская Федерация, МПК 7 С05С1/02, C05G 1/08. Способ получения стабилизированной аммиачной селитры / Наумов С. М., Коряков В.В., Кузнецов А.Г., Полякова О.А., Соловьев Б.А., Гарин Ю.М.; заявитель и патентообладатель АО “Минудобрения”. – №2000123962/12; заявл. 18.09.2000; опубл. 10.05.2002.
20. Пат. 7147830 Соединённые Штаты Америки, МПК 7 С 01 С 1/18. Стабилизированные гранулы нитрата аммония / Stabilized ammonium nitrate
granules / Kemira Growhow Oyj, Hero Heikki, Poukari Juhani. Jianzhou Wu; заявитель и патентообладатель Kemira Growhow Oyj (Finland). – № 11/206875;
заявл. 19.08.05; опубл. 12.12.06.
21. Колесников В.П., Москаленко Л.В. Термографические исследования модификационных превращений удобрения, полученного на основе аммиачной селитры // Химическая промышленность сегодня. – 2006 – № 7. – С. 18-21.
22. Brockel U., Wahl M., Kirsch R., Feise H.J. Formation and growth of crystal bridges in bulk solids // Chem. Eng. Technol. – 2006. – Vol. 29, № 6. – Р. 691-695.
23. Komunjer L., Affolter C. Absorption – evaporation kinetics of water vapour
on highly hygroscopic powder: case of ammonium nitrate // Powder Technol. – 2005. Vol. 157, №1. – Р. 67-71.
24. ГОСТ 21560.2-82 Удобрения минеральные. Метод определения статической прочности гранул. – ИУС.: – 2003 – С. 18-21.
25. Петропавловский И.А., Андриянова Е.А., Соколов В.В., Почиталкина И.А. Определение статической прочности гранул минеральных удобрений // Мир серы, N, P и K. – 2012. – Т. 6, – С. 8-13.
26. Kodirov B.X., Tojiyev R.R. Nitrat ammoniy suyuqlanmasiga glaukonit mineralini qo’shish asosida mikroelementlarga boyitilgan kompleks azotli mineral o’g’it olish. Scientific-technical journal (STJ FerPI, ФарПИ ИТЖ, НТЖ ФерПИ, 2022, Т.26.спец.выпуск №9), 195-198.
27. Колесников В.П., Москаленко Л.В., Белоусова Ю.Е. Влияние инертной добавки (цеолита) на прочность аммиачной селитры // Сб. тр. Общеросс. науч. – технич. конф. «Новые технологии в азотной промышленности». Ставрополь: СевКавГТУ, 2003. – С. 26-29.
28. Намазов Ш.С., Курбаниязов Р.К., Реймов А.М., Беглов Б.М. Прочность
гранул аммиачной селитры с добавками фосфоритов Центральных Кызылкумов // Химическая промышленность. – 2008. – Т.85, №2. – С. 65-70.
29. Leaper M.C., Bradley M.S., Cleaver J.A.S., Bridle I., Reed A.R., AbouChakra
H., Tüzün U. Constructing an engineering model for moisture migration in bulk solids as a prelude to predicting moisture migration caking // Adv. Powder Technol. – 2002. – Vol. 13, №4. – Р. 411-424.
30. 31 Таран Ю.А., Тaран А.В. Основные азотосодержащие минеральные
удобрения и технические решения для улучшения их качества // Изв. вузов.
Химия и хим. технол. – 2016. Т. 59, – №3. – С. 49-54.
31. Ботиров Б.Б., Беглов Б.М. Пути повышения качества аммиачной селитры // Хим. технол. Контроль и упр. – 2008. – №6. – С. 12-24.
32. Шведов К.К., Рубцов Ю.И. Оценка безопасности технологии получения
гранулирования и хранения аммиачной селитры // докл. на науч. – практ. конф «Современное состояние и проблемы производства аммиачной селитры»: ЗАО «Инфохим». – Спецвыпуск. – 2004. – С. 39-44.
33. Конвисар Л.В., Мошкович Е.Б. К вопросу обеспечения безопасной работы производства аммиачной селитры // Химическая промышленность. – 2002. – №6. – С. 40-42.
34. Жмай Л., Христианова Е. Аммиачная селитра в России и в мире. Современная ситуация и перспективы // Мир серы, N, P и K. – 2004, № 2. – С. 812.
35. Щеголев О.А. Состояние и проблемы производства аммиачной селитры //
Мир серы, N, Р и К. – 2004, – №2. – С.1-6.
36. Пат. 2416570 Российская Федерация, МПК С05С 1/18 (2006.01). Способ
получения водоустойчивого нитрата аммония (аммиачной селитры) / Михайдов Ю.М., Гатина Р.Ф., Хацринов А.И., Батырев А.В., Меркин А.А., Федотов П.И.; заявитель и патентообладатель Федеральное казенное предприятие «Государственный научно-исследовательский институт химических продуктов». – № 200912598/05; заявл. 06.07.2009; опубл. 20.04.2011.
37. Пат. 2480411 Российская Федерация, МПК С05С 1/18 (2006.01). Способ
получения водоустойчивого нитрата аммония / Михайлов Ю.М., Гатина Р.Ф.,
Хацринов А.И., Климович О.В.; заявитель и патентообладатель Михайлов
Ю.М., Гатина Р.Ф. – № 2010128039/05; заявл. 06.07. 2010; опубл. 27.04.2013.
38. Щеголев О.А. Состояние и проблемы производства аммиачной селитры
// Мир серы, N, Р и К. – 2004, – № 2. – С. 1-6.
39. Турдиалиев У.М., Намазов Ш.С., Реймов А.М., Сейтназаров А.Р., Беглов Б.М. Неслеживающаяся аммиачная селитра с добавкой бентонитовой глины Каттакурганского месторождения // Химический журнал Казахстана. – 2016. – №1. – С. 390-404.
40 Пат. 2491261 Российская Федерация, МПК С05С 1/00 (2006.01). Способ
получения неслеживающейся аммиачной селитры / Мухачева Т.Е., Медянцева Д.Г., Захарова О.М., Гуськова М.В., Зубарева Л.В.; заявитель и
патентообладатель ОАО «ЗМУ» Кирово-Чепецкого химического комбината. – №2012107181/13; заявл. 27.02.2012; опубл. 27.08.2013.
41. Kirilov PI. J. Non-caking fertilizers from ammonium nitrate and supplementary nutrients // Univ. Chem. Technol. And Met. – 2005. Vol. 40, №3. – Р. 209-212.
42. Нагалешкин Д.А., Гришаев И.Г., Долгов В.В. Исследование гигроскопичности смесей нитрата и сульфата аммония // Хим. технол. – 2011. -№10. – С. 593-597.
43. Kydyralieva A.D., Besterekov U.B., Petropavlovskiy I.A., Yermekov S.R. Thermal decomposition of ammonium nitrate: кinetics of the processes // V International Conference “Industrial Technologies and Engineering” ICITE – 2018. – Vol. 1. – Shimkent, 2018. – P. 212–216.
44. Махоткин И. А., Сахаров И. Ю., Махоткин А. Ф., Сахаров Ю. Н. Закономерности кинетики абсорбции аммиака азотной кислотой в условиях
производства аммиачной селитры // Вестн. Казанского техн. у-та. -2013. -Т. 16, №14. – С. 74 – 75.
45. Лавров В.В., Шведов К.К. О взрывоопасности аммиачной селитры и удобрений на ее основе // Научно-технические новости: ЗАО «ИНФОХИМ». – Спецвыпуск. – 2004. – №4. – С. 44-49.
46. Shen L., Wang X. Thermal stability assessment of antiexplosive ammonium
nitrate // Journal Univ. Sci. and Technol. Beijing. – 2005. – Vol. 1, №12. – Р. 12-15.
47. Соснии В.Г. Исследование взрывоопасности аммиачной селитры // докл. науч. – практ. конф. «Современное состояние и проблемы производства аммиачной селитры».: ЗАО «ИНФОХИМ». – Спецвыпуск. – 2004. -№2. – С. 5658.
48. Oxley J.C., Smith J.L., Rogers E., Yu M. Ammonium nitrate: thermal stability and explosivity modifiers //Thermochim. Acta. -2002.-Vol.384,№1-2. -Р.23-45.
49. Kydyralieva A.D., Besterekov U.B., Petropavlovskiy I.A., Nazarbeк U.B., Bolysbek A.A. Thermal decomposition of ammonia saltpeter: the process kinetics
and balance, mass calculations // V International Conference “Industrial Technologies and Engineering” ICITE – 2018. – Vol. 1. – Shуmkent, 2018. – P. 221226.
50. Besterekov U., Kydyralieva A.D., et al. Mass balance calculations of processes of ammonia saltpeter thermal decomposition and nitric acid absorption of ammonia // Bulletin of the Karaganda University. “Chemistry” series. – 2019. – Vol. 96. – P. 92-97. doi:10.31489/2019ch4/92-97.
51. Рубцов Ю.И., Казанов А.И. Скорость термического разложения твердого нитрата аммония в присутствии влаги и избытка азотной кислоты //Журнал прикладной химии. – 1990. – Т. 60. – №1. -С. 3-7.
52. Han Zh., Sachdeva S., Papadaki M.I., Mannan S. Effects of inhibitor and promoter mixtures on ammonium nitrate fertilizer explosion hazards // Thermochim. Acta. – 2016. – Vol. 624. – P. 69-75.
53. Tang Sh., Liu Z., Zhu G., Tong X., Lu Ch. Effect of additives on detonation
safety and heat stability of ammonium nitrate // Huafei Gongye. – 2003. – Vol. 30, № 4. – P. 28-32.
54. Buczkowski, D., Zygmunt, В., Pagowski, W. Effect of addition of inorganic substances on reduction of the explosibility of ammonium nitrate // Biuletyn Wojskowej Akademii Technicznej. – 2004. – Vol. 53, № 2-3. – P. 95-107.
55. Sun Jinhua. Catalytic effects of inorganic acids on the decomposition of ammonium nitrate / Sun Jinhua, Sun Zhanhui, Wang Qing-song, Ding Hui, Wang
Tong, Jiang Chuansheng // Journal Hazardous Materials. -2005.-№ 1-3 (127).-C. 204210.
56. Волкова А.В. Рынок минеральных удобрений – 2019 / Национальный исследовательский университет Высшая школа экономики Центр развития. –
2019. – 52 c.
57. РБК. Исследование рынков. Мировой и российский рынок минеральных
удобрений // http://marketing.rbc.ru
58. https://www.mordorintelligence.com/ru/industry-reports/ammonium-nitrate-market
